Determine the Molarity of a Solution
Molarity also means the same as molar concentration. Note our solvent could be distilled and carbon dioxide free water.
6 1 Calculating Molarity Problems Chemistry Libretexts
200 mol 400M solution.

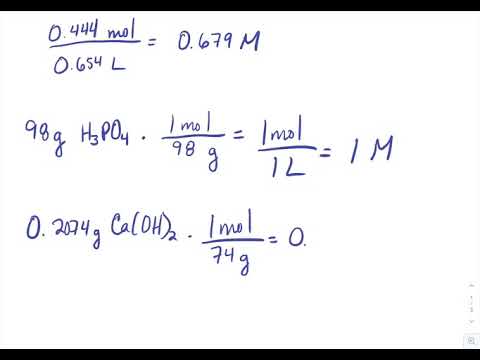
. Calculate the molarity of a solution prepared by dissolving 237 grams of KMnO 4 into enough water to make 750 mL of solution. 1st convert to moles 2nd plug into the molarity equation. Molarity concentration molar mass.
Grams active solute per liter of solution. Ppm 1 mg solute per liter of solution. Molarity refers to the concentration of a given solution.
Normality is similar to molarity except it expresses the number of active grams of a solute per liter of solution. The resulting quotient is the solution molarity in molL. Likewise a solvent is a substance in which another substance dissolves.
The Virtual Lab is an online simulation of a chemistry lab. More information and offline downloads. Next we will dilute this solution.
In case you have the ppm value repeat all the steps but substitute the density with the ppm and multiplying everything by 1000 mgg. This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles. Level 3- Given grams instead of moles and liters of solution.
From basic molarity formula we have obtained mole of solute NaOH is equivalent to one. What mass in grams of solid ammonium sulfate would be. If you need to calculate diluted molarity you can use the following formula.
In this question we have to find out the correct answer of given problem by the help of the Q. Find expected pH for a given concentration simply by entering the molarity or enter weight and total volume. You can also calculate the mass of a substance needed to achieve a desired molarity.
The table shows four solutions that were prepared by dissolving salt in water. Therefore when we use a solution dilution calculator it tells adding more water instead of salt then we will get. The lab allows students to select from hundreds of standard reagents aqueous and manipulate them in a manner resembling a real lab.
It is designed to help students link chemical computations with authentic laboratory chemistry. But when mixing a chemical solution you can determine the expected pH using well-studied well- documented stoichiometric theory. This last one works because the solution concentration is so low that we can assume the solution density to be 100 gmL.
You also need the concentrations of each ion expressed in terms of molarity or moles per liter or the means to obtain these values. Molarity mole of soluteliter of solution. To understand the topic as a whole you will.
Please scroll below to find our collection of. In chemistry a solution is a special type of homogeneous mixture composed of two or more substances. Then for an aqueous solution.
There are two individual formulas for both an acid solution and a basic solution. For acid solution n is the number of H ions given by a formula unit of acid and for a basic solution n is the number of OH- ions given by a formula unit of a base. Determine the molarity when 117g of NaCl are dissolved to make 0500 liters of solution.
For example an aqueous solution of sodium chloride NaCl aq with a molarity of 0154 mol L -1 or 0154 mol dm -3 could be represented as. Calculate the solubility product constant for leadII chloride if 500 mL of a saturated solution of leadII chloride was found to contain 02207 g of leadII chloride dissolved in it. 117g NaCl 1mol585g 200mol NaCl.
To estimate the molarity of any water solution. Ppm 1 mg solute per 1 kg solution. The valency is generally measured.
Solution - A solution is a mixture formed when a solid liquid or gaseous substance is homogeneously mixed with a liquid. 0486 g H2C2O4 mol H 2C O4 90036 g H2C2O4 2 mol KOH 1 mol H2C2O4 00108 mol KOH 6 Finally the molarity for potassium hydroxide is calculated as follows. This is the gram equivalent weight of solute per liter of solution.
Normality is often used in acid-base reactions or when dealing with acids or bases. Level 4-Given grams instead of moles and milliliters of solution instead of liters Determine. This article will provide you with the molarity definition and the molarity formula.
This example has neither the moles nor liters needed to find molarity so you must find the number of moles of the solute first. To learn more about Properties Types Videos. Divide it by the solutes molar mass in gmol.
Take the solutions density in gL. By definition its the number of moles of a solute or substance dissolved in a liter of a solution. Given Volume of solution 100 mL Molarity of MgNO32 solution 015 M 015 molL Density of Q.
The molarity of solution 1 is M_1moles_1 liter_1 and the molarity of solution 2 is M_2 moles_2 liter_2 Now rearrange the particular equations to determine moles. The pH calculator tool provides expected pH values for a variety of common laboratory and industrial chemicals. How to Calculate Normality of a Chemical Solution.
In such a mixture a solute is a substance dissolved in another substance known as a solvent. Note that we often use square brackets around the formula of solvated species to indicate the molarity of a solution the concentration of the solution in mol L-1. Also its this last modification of ppm the mgL one that allows us to go to molarity which has units of molL.
The normality of a solution is never less than its molarity. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved resulting in interactions that are specific to solvation. Molarity of KOH KOH mol KOH 001798L KOH 5 The mass of oxalic acid H2C2O4aq and Equation 4 are then used to determine the number of moles of potassium hydroxide present.
What is the molarity of a solution containing 0325 moles of solute in 250.

Molarity Chemistry Tutorial Youtube

How To Calculate Molarity Using Solute Mass Chemistry Study Com

Question Video Calculating The Molarity Of A Solution From Mass And Volume Nagwa

0 Response to "Determine the Molarity of a Solution"
Post a Comment